


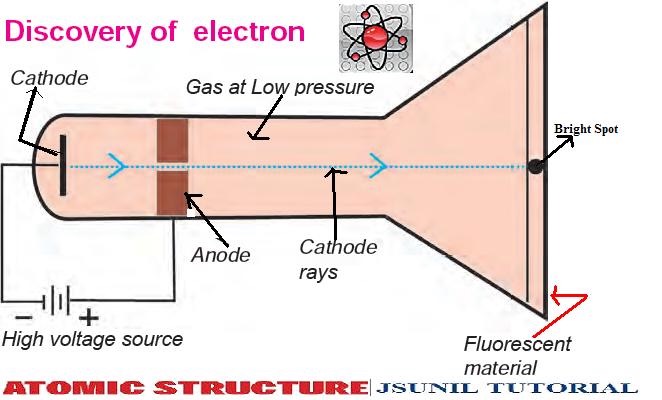
In 1884, he was appointed the Cavendish Professor of Experimental Physics and began his lifelong study of electromagnetism. In 1876, Thomson had received a scholarship to study at the University of Cambridge’s Trinity College, and four years later he graduated with a degree in mathematics. Thomson was a key player in establishing this new direction in physics. Little did scientists know that atomic theory was about to upend science and change our fundamental understanding of matter. Electricity was being tamed, and theories of thermodynamics were coalescing to explain the workings of steam engines. Thomson gave a lecture detailing his and others’ experiments with the energetic beams inside cathode-ray tubes.Īs the 19th century was coming to a close, many prominent thinkers believed that all of the great discoveries in science had already been made. The more I researched, the more I contemplated what it meant to discover something.Ĭathode rays, vacuum tubes, and the birth of atomic theoryĮxactly 125 years ago, the British physicist J.J. Many people, in fact, had a reasonable claim to aspects of the electron’s discovery. The challenge then was to find a museum artifact that captured that discovery.Ī Braun vacuum tube, like the one pictured at top, seemed like a good choice, because its inventor, Karl Ferdinand Braun, created it to study beams of electrons, and Thomson used a similar instrument for his experiments.īut as I dug into the histories of Thomson and Braun, I learned that theirs were two parallel stories involving many of the same players and similar outcomes (both men won a Nobel Prize in Physics), but having little else in common. For this month’s column, I knew that I wanted to write about the 125th anniversary of the electron’s discovery, which for simplicity’s sake I pegged to Thomson’s lecture. Of course, history is always more muddled than that. Thomson is often hailed as the discoverer of the electron based on that lecture 125 years ago. Corpuscles are electrons, and the plum pudding model gave way to Ernest Rutherford’s nuclear model in 1911. Thomson, who merely endorsed the idea.Ĭorpuscles and pudding are not how we think about the structure of an atom today. The model also became known as the Thomson model, although its chief proponent was William Thomson (Lord Kelvin), not J.J. In Thomson’s analogy, negatively charged corpuscles were like raisins suspended in a positively charged cake, resulting in a neutral atom. This model of the atom became known as the “plum pudding” model, so named for the popular English dessert. Thomson described his experiments with cathode rays to verify the existence of these subatomic corpuscles. “The atoms of the ordinary elements are made up of corpuscles and holes, the holes being predominant,” he continued. Thomson, during a lecture at the Royal Institution in London, on 30 April 1897.
C) that electrons were much smaller than alpha particles.“We shall call such particles corpuscles,” announced the physicist J.J. B)that atoms were too small to affect alpha particles.

What did Rutherford discover that Thomson did not understand?Ī) that an atom's electrons took up as much space as its nucleus. His discovery led to more detailed models of the atom, including the one that we use today. Thomson's discovery was important because he showed that the atom particle could be split into smaller particles. J.īeside above, why was JJ Thomson's discovery important? Importance of the Discovery. In Thomson's model, the atom is composed of electrons (which Thomson still called “corpuscles,” though G. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Keeping this in consideration, what was JJ Thomson's atomic theory? Thomson realized that the accepted model of an atom did not account for negatively or positively charged particles. He demonstrated that cathode rays were negatively charged. Thomson discovered the electron by experimenting with a Crookes, or cathode ray, tube.


 0 kommentar(er)
0 kommentar(er)
